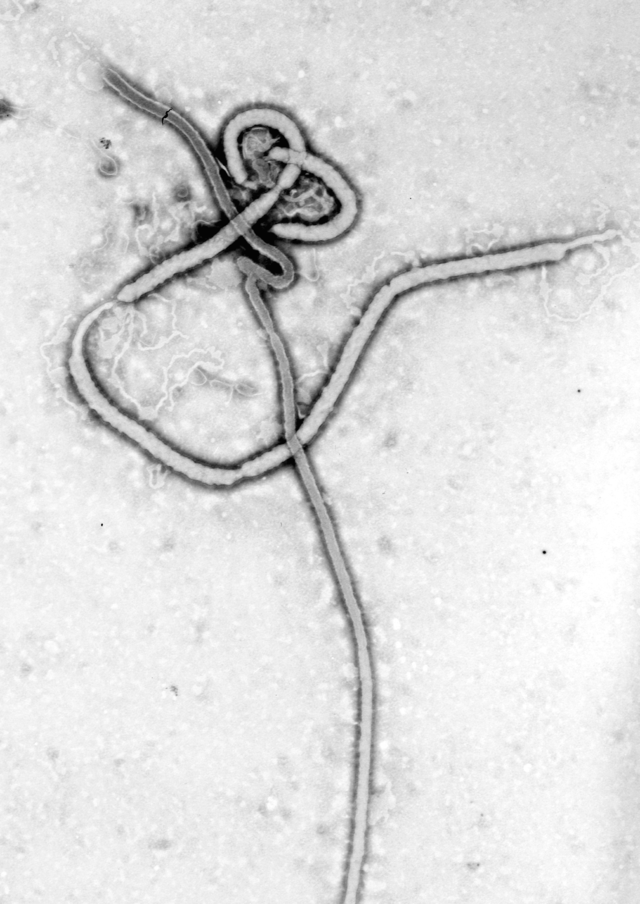
Between 6 and 22 June 1995, 8 patients in Kikwit, Democratic Republic of the Congo, who met the case definition used in Kikwit for Ebola (EBO) hemorrhagic fever, were transfused with blood donated by 5 convalescent patients. The donated blood contained IgG EBO antibodies but no EBO antigen. EBO antigens were detected in all the transfusion recipients just before transfusion. The 8 transfused patients had clinical symptoms similar to those of other EBO patients seen during the epidemic. All were seriously ill with severe asthenia, 4 presented with hemorrhagic manifestations, and 2 became comatose as their disease progressed. Only 1 transfused patient (12.5%) died; this number is significantly lower than the overall case fatality rate (80%) for the EBO epidemic in Kikwit and than the rates for other EBO epidemics. The reason for this low fatality rate remains to be explained. The transfused patients did receive better care than those in the initial phase of the epidemic. Plans should be made to prepare for a more thorough evaluation of passive immune therapy during a new EBO outbreak.
Between January and June 1995, Kikwit, a city 525 km southeast of Kinshasa, Democratic Republic of the Congo (DRC), was the epicenter of an outbreak of Ebola (EBO) hemorrhagic fever (EHF) [1]. During the epidemic, 316 cases of EHF were identified. The overall case fatality rate (80%; 249/311) [2] was comparable to rates observed during other epidemics (53% during the 1976 outbreak in southern Sudan, 88% during the 1976 Yambuku outbreak in DRC, and 53% during the February 1996 outbreak in Gabon) [3, 4].
Relatively little is known about the clinical manifestations of EBO virus infection or about the pathophysiologic mechanisms causing the disease [5]. During the previous EHF outbreak in Yambuku, very little had been done to organize patient care because most patients had died by the time the international medical team arrived [6].
There is no effective antiviral treatment for this very contagious and deadly disease. Ribavirin, an antiviral that is effective in patients with Lassa fever and possibly in patients with Crimean-Congo hemorrhagic fever, had no curative effect in monkeys infected with EBO [7, 8].
Following the Yambuku EHF epidemic, a great effort was made to collect serum from convalescent patients. Convalescent serum together with human interferon was given to a British researcher who became infected while working with EBO virus in the laboratory [9]. Viremia levels decreased rapidly after therapy was initiated, and the researcher survived. Whether this was because he received the convalescent serum is unknown. Convalescent serum does not neutralize homologous virus in standard virus neutralization tests, and in animal experiments, convalescent serum was not shown to be protective [9].
The treatment of EBO-infected patients consists mainly of palliative treatment and treatment to avoid cardiovascular collapse and renal insufficiency. During the initial phase of the EBO epidemic in Kikwit, very little patient care was offered. The priority was to stop the epidemic and to avoid further spread among health care workers. For this reason, protective equipment was distributed and invasive procedures were avoided. Infusions were rarely given, even if patients were dehydrated. The case fatality rate at that time was ∼80%. By the last phase of the epidemic, the situation had changed: Health care workers were trained in barrier nursing techniques, there were relatively few patients, and the epidemic was under control. When a nurse from Kikwit General Hospital, who had volunteered to care for EBO-infected Italian nuns, developed symptoms of EHF, the decision was made to give her a transfusion of total blood donated by a convalescent patient. (The infrastructure for doing plasma-pheresis was not available on site, and the convalescent serum that was collected in 1976 was not available [lost?]). After transfusion, the patient improved clinically; therefore, 7 other patients were treated in a similar manner. Herein, we describe the 8 EBO virus—infected patients who received blood transfusions from convalescent patients.
Patients and Methods
Between 6 and 22 June 1995, 8 patients (mean age, 33 years; range, 12–54) who met the criteria of the clinical EBO case definition used in Kikwit were transfused with blood donated by convalescent patients. Transfusions were given with patient consent or the consent of a family member if the patient had reduced consciousness.
Five convalescent patients, who had been discharged from the hospital between 2 and 15 May 1995, agreed to donate blood. All blood donors were negative for human immunodeficiency virus (HIV) infection as determined by an HIV spot test, and they were negative for hepatitis B surface antigen and rapid plasma reagin. Recipients and blood donors had the same blood group. In 4 blood donors, IgG and IgM EBO antibodies were detected but EBO antigen was not (table 1). The technique for detecting IgG and IgM EBO antibodies and EBO antigen is described in this issue by Ksiazek et al. [10].
Characteristics of 5 male survivors of Ebola infection who donated convalescent blood.
Blood was collected into a sterile plastic bag containing 63 mL of anticoagulant and transfused on the same day. Daily blood samples were obtained from patients to measure EBO antigenemia and EBO IgG and IgM antibodies. In all patients, a first blood sample was obtained prior to transfusion. All patients were treated at the isolation ward of Kikwit General Hospital.
Results
The characteristics of the transfusion recipients are summarized in table 2.
Characteristics of 8 Ebola-infected female convalescent blood transfusion recipients.
Patient 1
A 27-year-old nurse had been involved in the nursing care of 2 Italian nuns who died of EHF. She used protective clothing and gloves but may have touched her eyes inadvertently with contaminated gloves. On 30 May, she developed fever, headache, anorexia, and asthenia. She was treated with paracetamol, mebendazole, and chloroquine. On 1 June, the nurse developed diarrhea and vomiting, and the next day her high fever persisted (40°C), and she developed arthralgia and myalgia. Upon admission on 4 June, she complained of severe asthenia, abdominal pain (mainly in the right hypochondrium), myalgia, arthralgia, dysphagia, cough with bloody expectorations, and pruritus of the legs. She was too weak to stand or sit and had a temperature of 36.5°C. She was treated with oral rehydration solution, vitamin C, intravenous calcium, and papaverine. On 6 June, the nurse was transfused with 400 cm3 of blood. Two days later, her appetite improved, and the myalgia had disappeared; however the pain in her right hypochondrium and the asthenia persisted. Over the following days, her clinical condition continued to improve. During her illness, the nurse lost 10 kg of body weight. She was discharged from the hospital 21 days after admission.
Patient 2
A 12-year-old girl had been in physical contact with vomit from her pregnant aunt who died of EHF shortly after delivery of the baby. The girl had also carried and kissed the newborn baby, who died 3 days after birth; the baby was probably also infected with EBO. On 2 June, the girl developed a headache. Over the next days, fever and chills were followed by asthenia, abdominal pain, diarrhea, and dysphagia. On 11 June, she was admitted to the hospital with complaints of headache, diarrhea, abdominal pain, asthenia, vomiting, and fever. The girl also had a temperature of 40°C and a conjunctival injection, and she had difficulty opening her mouth.
The girl was treated with paracetamol, intravenous calcium, vitamin B complex, bicarbonate, butylhyoscine, and papaverine. Initially, her clinical condition deteriorated: She developed very severe asthenia, and the high fever continued. On 13 June, she was transfused with 150 cm3 of blood. The next day, she was treated with cotrimoxazole and chloroquine because of a temperature of 38.5°C and a nonproductive cough. On the following days, her symptoms gradually disappeared. During her hospitalization, the girl also received infusions of a solution of albumin, sodium chloride, and 10% and 50% glucose. During convalescence, she complained of arthralgia and myalgia, but by 21 June all of her symptoms had disappeared.
Patient 3
A 15-year-old girl had participated in the ritual funeral of her mother, who died of EBO on 27 May. On 2 June, the girl developed fever, chills, headache, vomiting, anorexia, asthenia, abdominal pain (mainly in the right hypochondrium), cough, and a conjunctival injection. On 9 June, the girl was admitted to the hospital with the same complaints and with dysphagia and a temperature of 38°C. She was treated with paracetamol, chloroquine, and cotrimoxazole. By 12 June, most of her complaints had disappeared; however, the asthenia and fever persisted, and the conjunctival injection did not disappear until 14 June. On 15 June, she was transfused with 150 cm3 of blood. During her hospitalization, the girl also received infusions of a solution of albumin, 5% and 10% glucose, and sodium chloride. Two days later, her temperature was normal. She continued to complain of a slight headache for ∼1 month, and was discharged from the hospital on 24 July.
Patient 4
A 54-year-old woman had been in contact with a patient who died of EBO in the maternity ward of Kikwit II Hospital. She had eaten from the same plate as the EBO patient, washed her hands in the same bucket, and slept on the rug that had probably been soiled by diarrhea stools of the EBO patient. One week later (4 June), she developed chills and fever. In the following days, she developed a headache, conjunctival injection, diarrhea, and vomiting.
She was admitted to the hospital on 11 June with complaints of severe asthenia, anorexia, generalized pain, dysphagia, fever, and diarrhea. A physical examination revealed a conjunctival injection and bleeding at an intravenous-injection site. She was treated with oral rehydration solution, quinine, intravenous calcium, cotrimoxazole, and vitamin K. The patient also developed a cough and abdominal pain (mainly in the right hypochondrium). On 13 June, she was transfused with 250 cm3 of blood. On 15 June, she still had serious asthenia, postprandial vomiting, cough, dyspnea, and abdominal pain in the right hypochondrium. Her symptoms progressively disappeared starting on 16 June. The patient complained of pain in her legs for 24 h. On 23 June, her temperature was normal; however, the fever reappeared on 26 June because of pneumonia. After 2 weeks of treatment with ampicillin, all her symptoms disappeared, and on 31 July, she was discharged from the hospital without any complaints.
Patient 5
A 44-year-old woman had cared for her daughter, who was hospitalized in a small clinic in Kikwit. A patient with EHF died in the bed next to her daughter. The woman had no direct contact with the EBO-infected person, but she had slept on a rug that had also been used by the EHF patient. The EHF patient had suffered from diarrhea and cough.
On 8 June, the woman developed headache, chills, asthenia, abdominal pain, and pain in her lower extremities, and on 12 June, she also developed diarrhea, anorexia, vomiting, and dizziness. She was admitted to the hospital on 15 June with severe asthenia, bloody diarrhea, abdominal cramps, and a temperature of 37.5°C; she did not have a conjunctival injection. The woman was treated with sodium chloride and glucose infusions, papaverine, and vitamin B. While hospitalized, she developed epistaxis, cough with hemoptysis, dysphagia, and a slight degree of obnubilation. On 22 June, her temperature was 40°C, and she had intense asthenia, continued to vomit, complained of dysphagia, and developed dysuria.
On 23 June, she was transfused with 250 cm3 of blood. Her clinical condition rapidly improved, and the next day the diarrhea and the vomiting stopped. On 25 June, however, she had urinary incontinence, complained of photophobia and of arthralgia at the maxillo-mandibular joints, and she had difficulty opening her mouth. On 26 June, a painful swelling of the left parotic gland was noted. On 27 June, her temperature returned to normal, and her clinical condition began to improve progressively. On 30 June, she was again able to open her mouth normally. During convalescence, the patient had several headaches. On 31 July, she was discharged from the hospital.
Patient 6
A 25-year-old woman, a daughter of patient 5, had been exposed to EBO virus under the same conditions as her mother. On 10 June, she developed chills, fever, nausea, headache, and anorexia, and a few days later, she developed diarrhea and severe asthenia. She was admitted to the hospital on 15 June, presenting with myalgia, arthralgia, watery diarrhea, vomiting, and severe asthenia. She also had a discrete conjunctival injection and was dehydrated, and abdominal palpation was painful, mainly in the right hypochondrium.
The woman was treated with atropine and metoclopramide. While hospitalized, she developed dysphagia, abdominal pain, lumbalgia, and arthralgia. On 19 June, she had decreased consciousness, anuria, and a high fever (40°C). She was treated with ampicillin and quinine. On 23 June, she was confused, had severe asthenia, anorexia, lumbalgia, and trismus, and was transfused with 250 cm3 of blood. The day after transfusion, she was comatose, in shock, and had urinary incontinence. She was treated with infusions (5% glucose and Ringer lactate), and on 26 June, she began responding to questions but remained confused. On 29 June, her temperature was normal, and she was neurologically normal except for a slight degree of trismus. On 1 July, she was able to walk, and on 4 July, she could open her mouth normally. During convalescence, the patient complained of arthralgia of both knees and the left shoulder. On 31 July, she left the hospital without any complaints but later developed uveitis.
Patient 7
A 40-year-old woman had been in physical contact with her nephew, who died of EHF. Her only contact was with his skin, due to a perforation in a glove she was wearing. On 12 June, she developed abdominal pain, diarrhea, and dysphagia. On admission to the hospital on 15 June, she complained of chills, headache, diarrhea, dysphagia, asthenia, anorexia, dizziness, and arthralgia. A temperature of 38°C and a discrete conjunctival injection were noted. She was treated with oral rehydration solution, paracetamol, quinine, dipyrone, and an infusion of 5% glucose. On 16 June, her temperature returned to normal, but the diarrhea became very severe (up to 14 stools/day). She was treated with norofloxacine. On 21 June, her fever reappeared (39°C). The diarrhea had stopped, but she developed thoracic pain without cough (increasing at inspiration and not dependent on the position of the patient) and her appetite had improved slightly. On 23 June, she was transfused with 450 cm3 of blood. The next day she vomited 3 times, had developed a nonproductive cough, and continued to complain of dizziness.
On 26 June, the patient complained of blurred vision, lumbalgia, abdominal pain, anorexia, asthenia, and chills. On 27 June, the patient had obnubilation, urinary incontinence, and tremors of the extremities. On 28 June, she was comatose, and neck stiffness was noted. She was treated with ampicillin (12 g intravenously), dexamethasone (15 mg), plasma expander, albumin, sodium chloride, and 10% glucose. During the following days, she progressively regained normal consciousness. On 2 July, she was neurologically normal but continued to suffer from severe asthenia. During convalescence, the patient complained of headache and polyarthralgia. On 31 July, she was discharged from the hospital.
Patient 8
A 48-year-old woman had been in physical contact with a friend who died of EHF. On 19 June, the woman developed chills, fever, headache, diarrhea, and dizziness. On 21 June, she was admitted to the hospital with diarrhea (with 8 episodes of melena), vomiting, headache, severe asthenia, high fever (39°C), and discrete dysphagia. Bleeding and a hematoma were noted at an intravenous-injection site, and she had a conjunctival injection. On 22 June, she was transfused with 400 cm3 of blood and treated with vitamin K, sodium chloride, and 10% glucose infusions. On 24 June, she had a generalized epileptic seizure. She did not have a fever, and she was treated with diazepam. Six hours later, she was found dead on the floor, next to her bed, with a hematoma on her face.
Evolution of EBO antigenemia and EBO IgG and IgM antibodies (table 3)
Clearance of Ebola antigens and development of Ebola IgG and IgM antibodies in infected patients who received transfusions of convalescent blood.
EBO antigens were detected in all transfusion recipients prior to transfusion. In 5 (83%) of 6 patients tested, EBO antigens had disappeared before day 4 after transfusion. In 4 (57%) of 7 transfusion recipients tested, EBO IgG or IgM antibodies were present before transfusion (only 2 of them had both IgG and IgM antibodies). After transfusion, IgG and IgM antibodies were detected in 7 (87.5%) of the 8 blood recipients. IgM antibodies were never detected in the 1 patient who died (patient 8).
Discussion
In 1995 during an outbreak of EBO virus in Kikwit, DRC, there was a 12.5% case fatality rate among 8 EHF patients who received blood transfusions from convalescent donors. This rate differs significantly from the overall case fatality rate (80%) for the epidemic [2] and from the case fatality rates for other epidemics [3]. The reason for this lower case fatality rate remains to be explained; however, it is known that the transfused patients received relatively better care (including infusions of glucose and electrolytes, treatment with antibiotic and antimalaria drugs, and food supplementation) than patients in the early stage of the outbreak. Two other EHF patients who were treated in Europe also survived [11, 12]; only 1 of them received convalescent serum. However, 6 other EBO-infected patients (Italian nuns) in Kikwit who were treated with infusions died.
Five other EBO patients (2 at the Mosango hospital and 3 at the Kikwit hospital) presented with bleeding manifestations and received blood transfusions from convalescent patients; 4 of them died. One of them was a 70-year-old nun who worked as a nurse at Kikwit General Hospital. Moreover, an EBO-infected Belgian nun who was transferred from Yambuku to Kinshasa in 1976 also died after having received blood transfusions from convalescent patients on days 5, 7, and 8 of her illness [13].
It has been suggested that at the end of an EBO epidemic, the virus may become less infectious and less virulent [14]. It is unlikely that this was the case in Kikwit. The 8 transfused patients had clinical symptoms similar to those of other EHF patients seen during the epidemic. All were seriously ill with severe asthenia; 4 presented with hemorrhagic manifestations; and 2 became comatose as the disease progressed. Moreover, sequencing of EBO isolates obtained at different times during the epidemic showed that the virus was genetically stable during the Kikwit epidemic (all sequences were identical) [15]. In addition, in other EBO outbreaks, no genetic variability was detected between virus samples obtained during each outbreak [16].
The 8 transfused EBO-infected patients in this report had not been in contact with EHF patients early in the epidemic. Therefore, it is unlikely that a reduced virulence at the end of the outbreak was caused by an acquired immunity of an unknown nature by persons who were exposed early in the outbreak to a low dose of defective virus. Evidence from animal studies indicates that EBO-specific immunoglobulin of equine origin has some activity in suppressing viremia and delaying disease onset in and death of nonhuman primates [17, 18]. However, effective treatment of human patients may require antibodies with higher specific activities and more favorable pharmacokinetic properties than the presently available equine IgG [19].
This study suggests that with adequate symptomatic treatment of EHF patients, the EBO case fatality rate may drop significantly, as seen with other life-threatening infectious diseases, such as cholera and dengue. Transfusions are probably useful for the treatment or prevention of shock and may provide coagulation factors to stop or to prevent bleeding. However, because of the small number of patients studied and the lack of control subjects, we cannot conclude that the neutralizing antibodies in transfused convalescent blood improves the outcome for EHF patients.
Because the reservoir or vector of the EBO virus is not known, it is likely that in the future, there will be more EBO epidemics of EHF. It is hoped that the lessons learned during the Kikwit outbreak will enable us to reduce case fatality rates. Additional animal experiments with passive immune therapy are certainly needed; however, in the absence of an effective antiviral, plans should also be made to prepare for a more thorough evaluation of the efficacy of hyperimmune plasma treatment during a new EBO virus outbreak.
- © 1999 by the Infectious Diseases Society of America
- K. Mupapa,
- M. Massamba,
- K. Kibadi,
- K. Kuvula,
- A. Bwaka,
- M. Kipasa,
- R. Colebunders and
- J. J. Muyembe-Tamfum on behalf of the International Scientific and Technical Committee
+Author Affiliations
- Reprints or correspondence: Dr. R. Colebunders, Institute of Tropical Medicine, Nationalestraat 155, B-2000 Antwerpen, Belgium (bcoleb@itg.be).
Source : Oxford Journals












































Facebook
Twitter
Pinterest
Google+
RSS